The Future of
Plasma Fractionation
Has Arrived
Watch as Aegros HaemaFrac® uses the mobility of ions in an electric field to transport proteins across a membrane into a collection chamber. This physical arrangement enables the selective isolation of high yield, high purity plasma proteins in rapid time.
Electrical ions have different migration rates depending on their total charge, size, and shape, and can therefore be targeted for separation.
We use buffers to change the charge on these ions. Using pH we can regulate the charge on these ions to positive or negative, providing the mechanism for separation.
For example, a negative charge is added to a molecule, so it moves towards the positive electrode. This technique has been used as a laboratory tool, called gel electrophoresis, for over 100 years. Many people have tried unsuccessfully to scale up from analytical gel electrophoresis to a large or preparative scale. Aegros HaemaFrac® is first preparative electrophoresis process to be approved for fractionation of a therapeutic product.
This technique is used particularly for macromolecules, such as proteins.
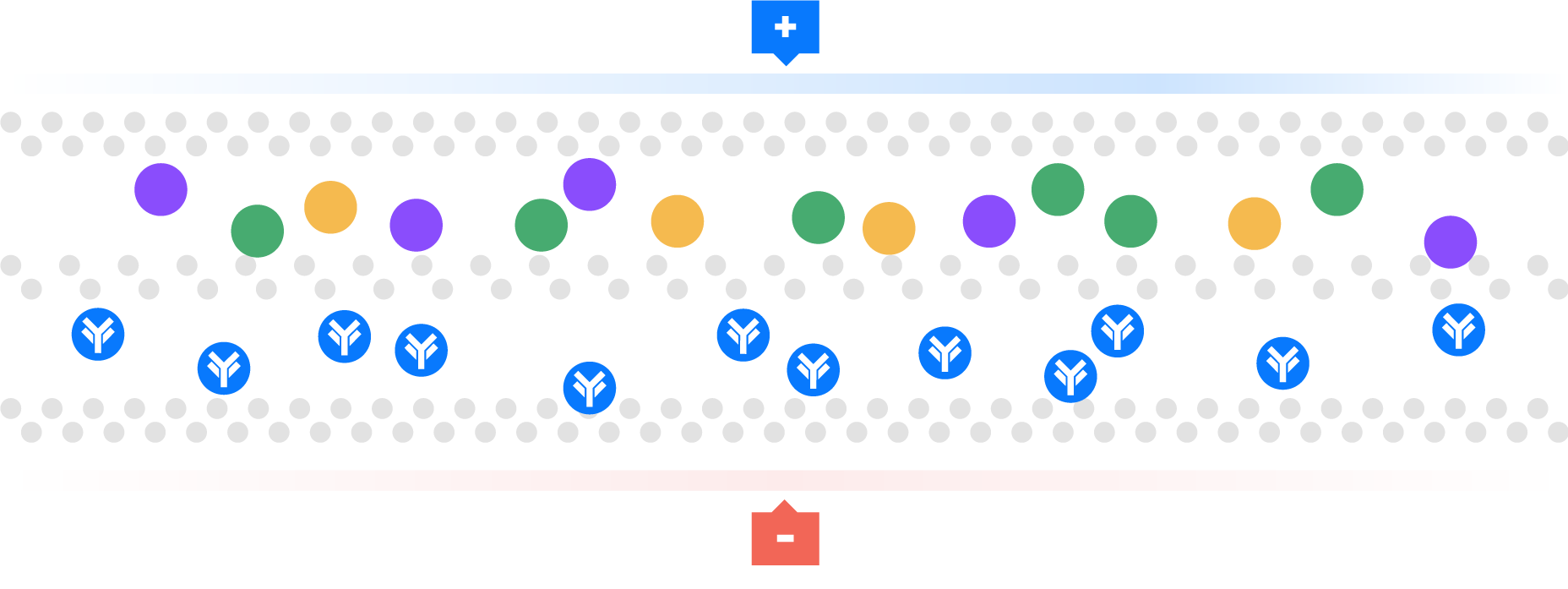
Aegros HaemaFrac® is the result of more than 40 years developmental work. Dr Joel Margolis conceived the idea in the late 1970’s with an initial working prototype produced in 1984. The first use in plasma fractionation was developed by Drs Nair and Wang in the form of Tangential Flow Electrophoresis in the late 90’s. They set up PrIME Biologics in Singapore which became the first cGMP accredited plant in the world using an HaemaFrac® capture step in 2016.
Hyperimmune fractionation is particularly sensitive to process yields and product activity. Aegros HaemaFrac® process is a gentle process which produces a high yield high activity product. This is particularly important when there is a limited amount of the hyperimmune plasma as is the case with Covid-19 convalescent plasma. Aegros HaemaFrac® process provides approximately twice the amount of therapeutic product from a given litre of convalescent plasma. This is way we say Aegros HaemaFrac® is the ‘micro processor’ compared to ‘valves’ for plasma fractionation.
The biopharmaceutical industry has evolved from microbial production of simple proteins such as insulin and somatotropin in the 1980’s to sophisticated systems for production of recombinant therapeutic proteins such as human FVIII. While the industry has witnessed major innovation in upstream (cell culture) bioprocesses, there has been little innovation in the downstream processing (plasma fractionation).
Aegros has extensive intellectual property, including provisional patents, covering its HaemaFrac® and associated processes.

Aegros HaemaFrac®
Significantly reduces production steps compared to current technology thereby reducing manufacturing costs;
Eliminates the use of ethanol or other solvents. This provides significant environmental, safety and cost savings;
Uses electrical charge to selectively isolate plasma proteins with a high yield, purity and activity while providing increased protection against pathogen contamination.
Aegros HaemaFrac® process uses the mobility of ions in an electric field to transport proteins across a membrane into a collection chamber. This physical arrangement enables the selective isolation of high yield, high purity plasma proteins in rapid time.
Electrical ions have different migration rates depending on their total charge, size, and shape, and can therefore be separated. We use buffers to change the charge on these ions. Using pH we can regulate the charge on these ions to positive or negative, providing the mechanism for separation.
The technique is used particularly for macromolecules, such as proteins. For example, a negative charge is added to a molecule so it moves towards the positive electrode. This technique has been used as a laboratory tool, called gel electrophoresis, for over 100 years. Many people have tried unsuccessfully to scale up from analytical gel electrophoresis to a large or preparative scale. Aegros HaemaFrac® is first preparative electrophoresis process to be approved for fractionation of a therapeutic product.
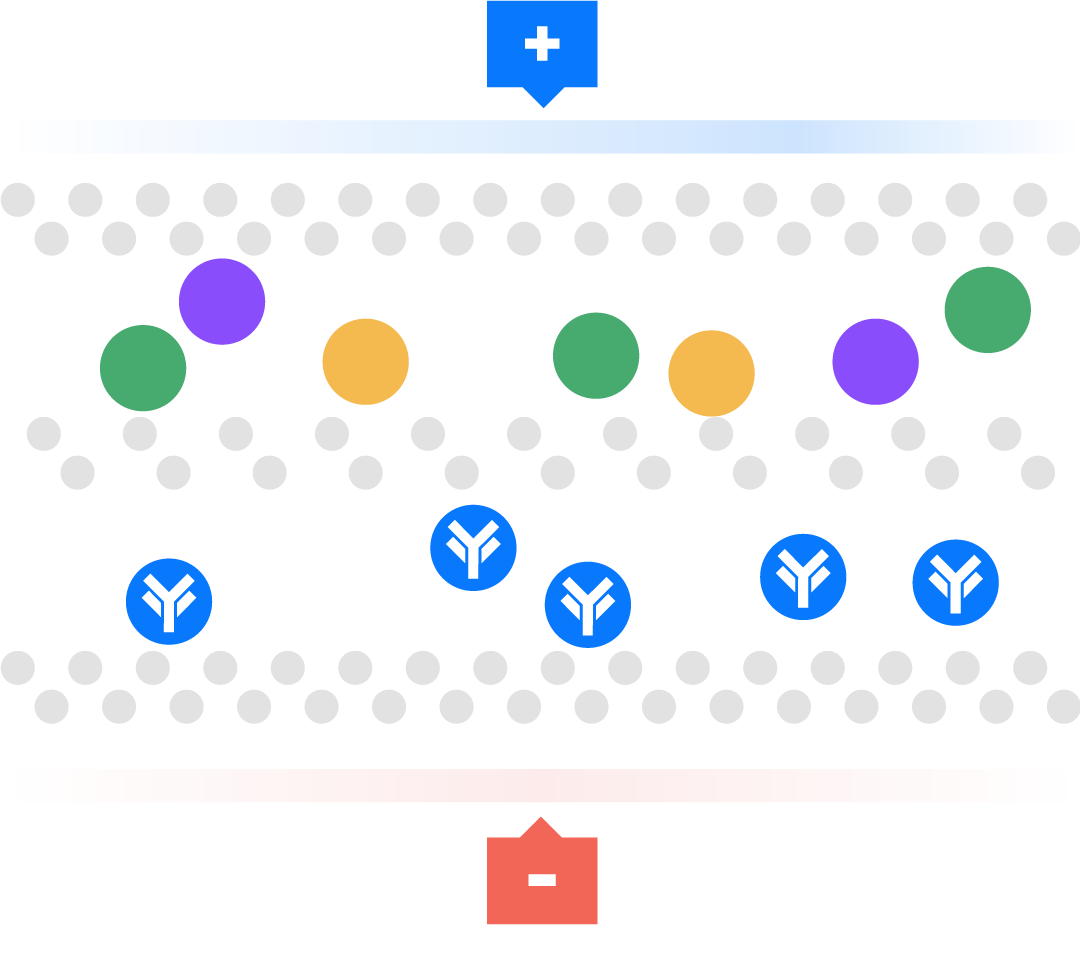
Aegros HaemaFrac® is the result of more than 40 years developmental work. Dr Joel Margolis conceived the idea in the late 1970’s with an initial working prototype produced in 1984. The first use in plasma fractionation was developed by Drs Nair and Wang in the form of Tangential Flow Electrophoresis in the late 90’s. They set up PrIME Biologics in Singapore which became the first cGMP accredited plant in the world using an HaemaFrac® capture step in 2016.
Hyperimmune fractionation is particularly sensitive to process yields and product activity. Aegros HaemaFrac® process is a gentle process which produces a high yield high activity product. This is particularly important when there is a limited amount of the hyperimmune plasma as is the case with Covid-19 convalescent plasma. Aegros HaemaFrac® process provides approximately twice the amount of therapeutic product from a given litre of convalescent plasma. This is way we say Aegros HaemaFrac® is the ‘micro processor’ compared to ‘valves’ for plasma fractionation.
The biopharmaceutical industry has evolved from microbial production of simple proteins such as insulin and somatotropin in the 1980’s to sophisticated systems for production of recombinant therapeutic proteins such as human FVIII. While the industry has witnessed major innovation in upstream (cell culture) bioprocesses, there has been little innovation in the downstream processing (plasma fractionation).
Aegros has extensive intellectual property, including provisional patents, covering its HaemaFrac® and associated processes.

Aegros HaemaFrac®
Significantly reduces production steps compared to current technology thereby reducing manufacturing costs;
Eliminates the use of ethanol or other solvents. This provides significant environmental, safety and cost savings;
Uses electrical charge to selectively isolate plasma proteins with a high yield, purity and activity while providing increased protection against pathogen contamination.
Aegros HaemaFrac® significantly reduces production steps compared to current technology.
Aegros’ process connects to the front and back end of the Cohn process replacing the multiple capture steps with a single capture step. This increases overall process yield, reduces process time, steps and cost making plasma fractionation viable at the 50,000L level.
Aegros HaemaFrac® significantly reduces production steps compared to current technology.
Aegros’ process connects to the front and back end of the Cohn process replacing the multiple capture steps with a single capture step. This increases overall process yield, reduces process time, steps and cost making plasma fractionation viable at the 50,000L level.
Current fractionation process
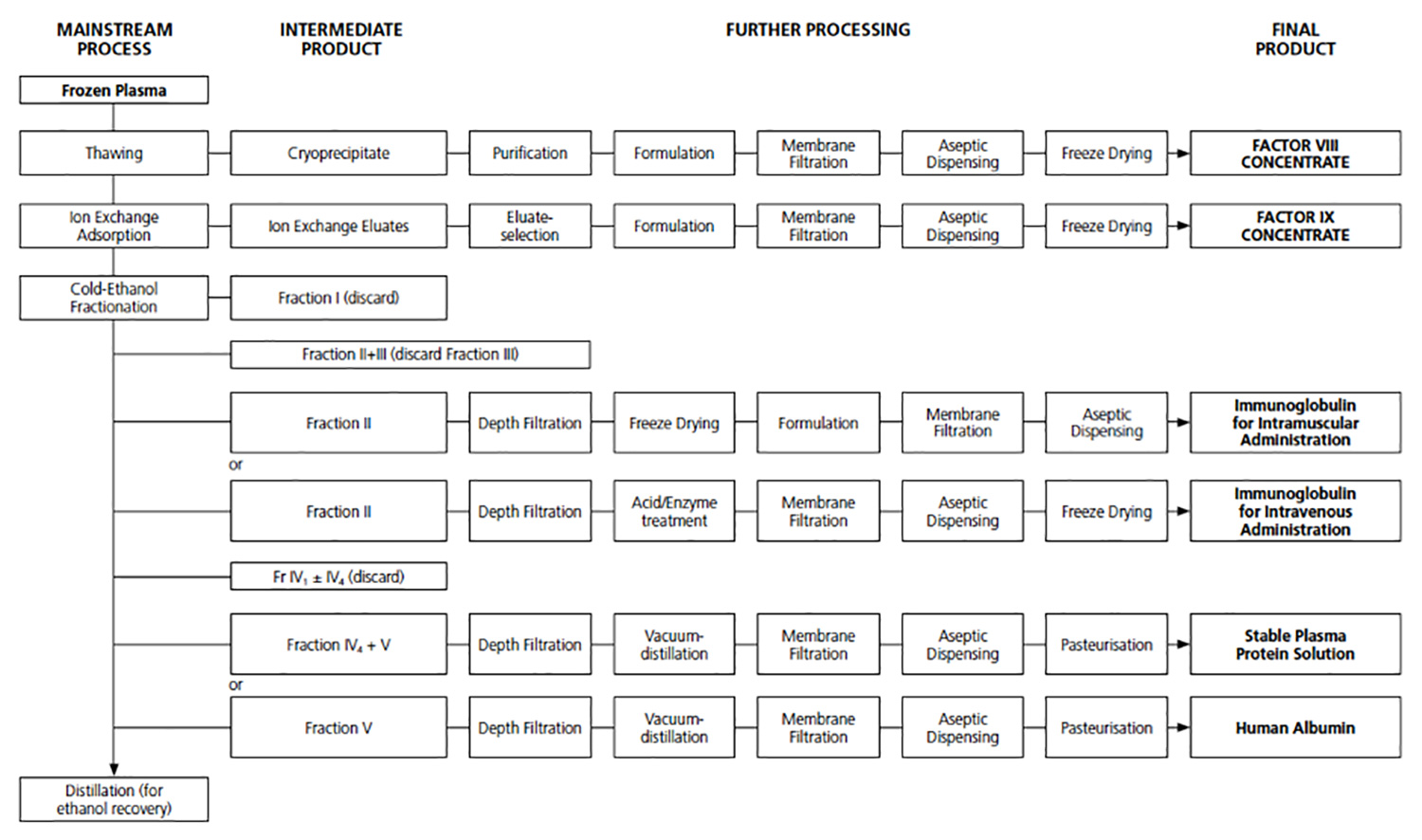
The current fractionators have evolved their processes from the original Cohn process developed by Dr Edwin J Cohn, first used in December 1941 at Pearl Harbor. While incremental improvements have been made the backbone of this process has remained the same over the ensuing 75 years.
Existing fractionators manufacture a number of products from human plasma, generally in a cascade process. Below are a number of examples showing these cascade fractionation processes.
All of these processes start with human plasma and follow a significant number of production steps to produce at least three products, Albumin, IVIG & FVIII. Each of these products are linked in a cascade fashion, such that a change in the production process of one product will impact on the production of the next product. Each production step introduces product losses, cost and time.