FAQ
You may have questions about aspects of Aegros' growth, strategy, and operations.
Here are answers to some of the most common questions we are asked.
In Australia there is only one purchaser of blood plasma medicines, the National Blood Authority (NBA). The NBA’s 2021-2022 Annual Report lists total purchases of blood plasma medicines totaling $762.2m. This market can be further segmented into recombinant and Plasma Derived Medicines (PDM). The market which Aegros address is the PDM and that represented $548.9m in the 2022 year.[1]
Demand for PDM’s is growing annually. The greatest growth was for Immunoglobulins, as shown in the table below.
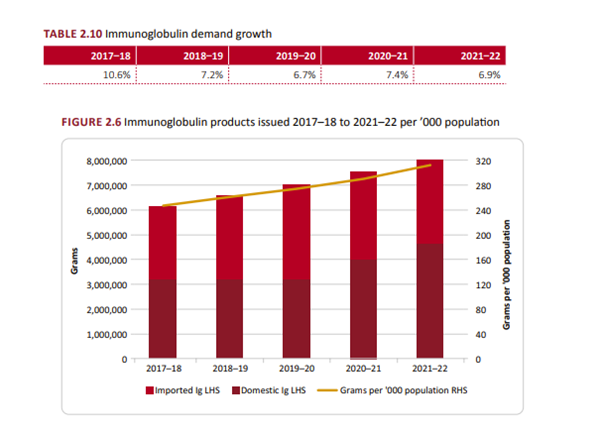
[1] See NBA 21-22 Annual Report, page 38.
Self-sufficiency in PDM’s is an essential part of any nation's sovereignty, particularly when you are an island at the end of a long transport link, as Australia is.
If Covid-19 has taught us anything it is that access to life saving drugs is critical and countries will restrict the shipment of drugs in times of need. Australia is currently exposed as 42%[1] of the PDM’s used in 2022 was imported.
Aegros' HaemaFrac® will enable Australia to achieve Structural Resilience. If Aegros was to fractionate all of the plasma collected by Lifeblood in 2022, it would have enabled Australia to achieve 100% self-sufficiency, helping Australia increase its structural resilience.
PDMs are lifesaving as they are used every day in procedures such as trauma surgeries and organ transplants. They are also critical to ensuring the quality of life of around 3-10% of Australians who are immunocompromised, and who rely on these medicines.
[1] See NBA 21-22 Annual Report, page 49.
All therapeutic products in Australia are regulated by the Therapeutic Goods Administration (TGA).
Before a therapeutic product can be sold into the Australian market it has to be included by the TGA on the Australian Register Therapeutics Goods (ARTG).
Before the TGA adds a therapeutic product to the ARTG it requires clinical data supporting the clinical need and safety of the proposed drug. This generally takes the form of a clinical trial and other clinical data to show the relevance, safety and saving to the Australian community.
For most drugs there is another step call the Pharmaceutical Benefits Scheme (PBS). Once a drug is registered on the PBS it means the Government will pay. In the case of PDM’s these are assessed by the National Blood Authority (NBA).
Supply of PDM’s has been an issue ever since these drugs were introduced post WWII. The initial codification of these regulations occurred when the Government privatised CSL in 1994. Then in 2003 the Federal Government passed the National Blood Authority Act 2003, which established the NBA. Finally, in 2010 the World Health Organisation (WHO) issued resolution WHA63.12, which calls for member countries to achieve self-sufficiency in plasma derived medicines.
The National Blood Authority Act 2003 states that the NBA is:
“to carry out national blood arrangements to ensure that there is a sufficient supply of blood products and services in all the States and covered Territories; and to carry out national blood arrangements relating to safety measures, quality measures, contingency measures and risk mitigation measures for the supply of blood products and services.”
The NBA is the sole entity which controls the collection of plasma in Australia and the purchase of PDM’s for use in Australia. As part of these functions the NBA also has advisory groups who advise the NBA on best clinical practice including new treatments.
The NBA has a contract with Lifeblood to pay for the collection of plasma in Australia. The NBA also has an exclusive contract to supply CSL with all of the plasma collected by Lifeblood. Further, the NBA contracts with other PDM suppliers to provide the additional PDM’s which cannot be supplied from the plasma collected by Lifeblood.
In 2021 Lifeblood collected 819,000L of plasma, which was supplied to CSL. CSL processed this plasma into PDM’s and in the case of immunoglobulins (IVIG) supplied approximately 54% of the immunoglobulins used in that year. The balance of 46% came from plasma sourced outside of Australia.
In 2021-22 the NBA purchased approximately $1,475m[1] of blood & blood products. Of these $708m related to the purchase of plasma from Lifeblood and $548m related to the purchase of PDM’s.
In 2020-21 the NBA paid CSL $57.14 /g[2] for the IVIG fractionation from the Australian plasma collected by Lifeblood and $45/g for the overseas collected plasma. The product manufactured from Australian plasma does not include the cost of the plasma, whereas the overseas supplied product does include the cost of plasma. Plasma represents approximately 50% of the total cost.
[1] NBA Annual Report 2021-22 Page 38.
[2] NBA Annual Report 2020-21 Page 155.
Lifeblood does a wonderful job collecting plasma from the Australian population.
In 2021-22 it collected just over 819,000L [1] of plasma for fractionation. This is world's best practice for a non-remunerated volunteer donation country (by comparison over 65% of the plasma collected in the world comes from America where they pay for plasma donations).
And yet, this worlds' best practice by Lifeblood is let down by the inefficiencies of the existing 90-year-old Cohn fractionation process.
To put this into context, approximately 50% of every non-remunerated donation is wasted/lost in the production process. We owe it to the volunteer donors to respect their societal contribution by maximising the lifesaving drugs we can extract from this plasma.
This is why Aegros developed the HaemaFrac®.
[1] See NBA 21-22 Annual Report, page 42.
This method of processing employs more than 10 steps of adding alcohol to plasma and purifying precipitates [1].
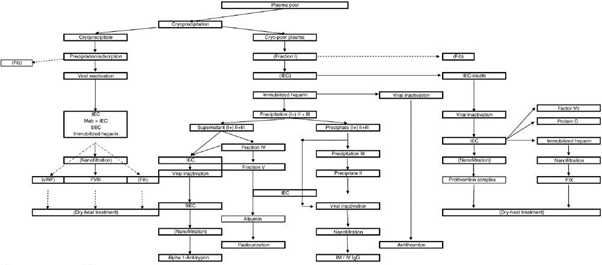
At each stage of the process, a little bit of the valuable protein gets lost due to a range of factors. By comparison, the HaemaFrac® provides a single capture step which means a 4-step total manufacturing process.
Cohn fractionation is expensive to set up, is energy intensive, uses volatile organic solvents, heat, and pressure. It is usually coupled with chromatography which again is expensive because of the specialised resins used.
The combination of the multiple steps and the none-too-gentle manner of Cohn processing results in a yield of at best 58% of the proteins from the donated plasma. This means the destruction or waste of around 42% from each litre of donated plasma – nearly half!
Due to Cohn fractionation resulting in the waste of nearly half of the valuable proteins in donated plasma, Australia is unable to significantly supply PDMs to reach self-sufficiency. New treatments are harder to develop as demand far out strips supply.
More information on Cohn processing is available.
[1] Thierry Benouf Modern Plasma Fractionation, Transfusion Medicine Review 2007 pp101-117.
Medicines made from blood plasma are life-saving therapies that are an indispensable part of modern medicine.
Beyond directly saving lives in hospital surgeries, plasma medicines ensure quality of life for patients with a wide range of illnesses such as:
- Acquired hypogammaglobulinaemia
- Chronic inflammatory demyelinating polyneuropathy (CIDP)
- Primary immune deficiency (PID)
- Myasthenia gravis
- Inflammatory myopathies
- Multifocal motor neuropathy
- Secondary hypogammaglobulinaemia
- Haemophilia, and
- A range of neurological conditions.
New therapies are constantly emerging for these and other ailments and demand in Australia is growing at around 7% a year.
Aegros' first product is a Covid-19 hyperimmune.
This product is designed to provide passive immunity to those people for whom vaccination is less effective. This includes people who are immune challenged and those undergoing chemotherapy.
Aegros would like to source its blood plasma from Australian donors so that we can lift Australia’s self-sufficiency from around 58% to 100%.
Aegros is engaging with the National Blood Authority in order to use HaemaFrac® technology to lift Australia’s self-sufficiency and to save money for the Australian taxpayer each and every year.
Right now, Lifeblood does not collect Covid-19 convalescent plasma. So, Aegros is sourcing its plasma from the United States, which supplies around 65% of the world’s blood plasma.
The HaemaFrac® can extract any protein from the around 3,000 contained within circulating blood plasma.
Aegros anticipates that traditional plasma medicines such as immunoglobulin and albumin will be the mainstay of our outputs, representing about 80% of future business.
The HaemaFrac® can fractionate any of the proteins in plasma. Aegros will work with the local Government entities to determine what other proteins they wish to purchase. These could include clotting factors such as Factor VIII, Factor IX, Factor XII, and fibrinogen.
Aegros’ HaemaFrac® is currently producing and will continue to produce speciality products. Right now, that takes the form of our hyperimmune against SARS-CoV-2 which is currently in clinical trial but in the future will include other hyperimmunes, new immunoglobulin therapies, Anti-D, and plasminogen.
Watch this video for detailed information about Aegros’ commercial scale-up.
Aegros has been using its HF-01 HaemaFrac® to manufacture Covid-19 hyperimmune in its TGA GMP licensed facility since 2021. This system has operated at an annual 30,000L capacity.
In June ’23 Aegros closed this facility and commenced renovations to lift the capacity to 100,000L pa. As part of this renovation Aegros has ordered an 8 cartridge HaemaFrac® system. Aegros expects this renovation to be completed by the end of the ’23 calendar year. Aegros will then ask the TGA to audit the new facility for the commercial manufacture of its Covid-19 hyperimmune.
As an aside, we understand Aegros HaemaFrac® facility is the only exclusive hyperimmune facility in the world. This is important as Aegros is the only organisation which can pivot as and when the next pandemic strikes. Aegros can manufacture a new hyperimmune in a matter of weeks.

Did the answers provided on this page provide you with the information you were looking for?
If you have a question that was not answered here, please let us know and we will do our best to answer it.