From the Founders
As of next month it will be 38 years since we started this journey, in May 1985. While that may appear to be an exceptionally long time, it talks to the tenacity, complexity and tortuous path one has to travel to bring a new technology to commercial reality. Today we can say we have a line of sight on our first commercial sales – later this year/early in 2025. This is more than a milestone, it is the realisation of an innovative spark by Joel and Perry in 1985, turned into commercial sales by Hari and myself. It will be a moment to call ‘MISSION ACCOMPLISHED’.

As we have said on many an occasion, an IPO is not a goal but the result of Aegros achieving its goals. In
light of reaching our ‘Mission Accomplished’ moment, we have resolved to press the button on Aegros’ IPO.
The Road to IPO
We believe this ‘Mission Accomplished‘ moment is less than 12 months away, and for that reason have agreed to press the button on the IPO process.

This is not a trivial process and will involve selecting a stock exchange, appointing lead brokers, selecting board members, due diligence process and preparing a prospectus to name a few items. We will keep shareholders informed as this process evolves.
Share Purchase Plan
In light of the fact the Board has resolved to commence the IPO process, it has resolved to offer our existing shareholders an opportunity to participate in a capital raising before the IPO, in what will probably be the last opportunity.
We are looking to reward our shareholders for supporting the Company through its earlier higher risk phase by offering this Share Purchase Plan (SPP) with attached bonus shares. The details of this are contained in the SPP Booklet attached to this mailout. We encourage you to reach out to either Andrew
Iliadis at Aegros or Sean Sandilands at STK if you have any questions. Their contact details are in the offer document.
SPP Documents
- Share Purchase Plan Offer Booklet
- Share Purchase Plan Application Form
- Share Purchase Plan Q&A Letter
Clinical Trial Update
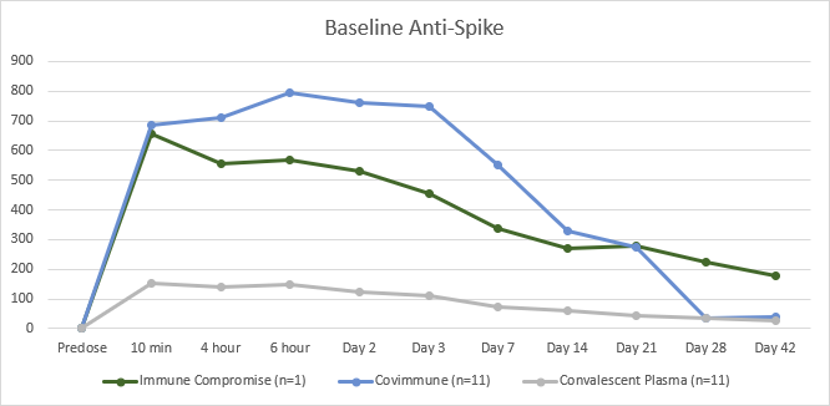
Both arms of the clinical trial for the establishment of passive immunity against Covid-19 is now complete.
Both arms showed significant rises in antibody levels with the second arm showing four times more antibodies than the convalescent group. The trial also allowed the infusion of one immunocompromised patient. The exciting result here was that the patient with zero Covid anitbodies had antibody levels similar to healthy subjects after infusion of the Aegros hyperimmune and this effect lasted for nearly 42 days. While this is an observation from only one patient it shows the potential for using hyperimmunes as a form of establishing passive immunity for immunocompromised patients.
$65m Haemafrac® Facility Upgrade
Once the clinical trial batches of our Covid-19 Hyperimmune were manufactured, our Engineering team turned its attention to designing a 100,000L state-of-the-art-facility. Thanks to input from the TGA we’ve included electronic batch records (EBR) that reduces the human element, increasing efficiency, safety & accuracy while reducing cost.
Demolition started in December and construction began soon thereafter. By February we started to see the clean rooms being constructed and in late March the first rooms for the manufacture of our Haemafrac® cartridges was handed over to Operational teams.
Since then, the Haemafrac® has been moved into place and the water for injection (WFI) facility has been turned on. As at today this project is some 65-70% complete. The next areas to be worked on are the sterile filling and then we will move into the QC labs. We expect this facility to be completed by the end of June.
You can watch a video of the construction activities below.
First Sales
Sales of our Covid-19 Hyperimmune is a two step regulatory process.
One: the $65m Haemafrac® facility will undertake a GMP audit. The GMP license enables Aegros to manufacture its Covid-19 Hyperimmune.
Two: Product approval will come at the end of 2024/early 2025 when the TGA lists Aegros Covid-19 Hyperimmune on the Australian Register of Therapeutic Goods (ARTG). From this point Aegros can sell its Covid-19 Hyperimmune in Australia.
Between these steps, Aegros can utilise its HaemaFrac® facility by toll manufacturing therapeutic plasma products for a number of less developed countries, starting in August/September this year. Not only will this help optimise the Haemafrac® process, it will also generate sales, reducing the cash burn.