Patients first
through innovation
Transforming the lives of patients with a safe, secure, sustainable supply of therapeutic plasma products
Aegros is Latin for patient. Every day we strive to place the patient first by providing therapeutic plasma products they would not otherwise be able to access.
At Aegros we believe access to life saving therapeutic plasma drugs is a human right and not a privilege.
Aegros Board

Founding Executive Chair
Professor Hari Nair
Professor Nair graduated with honours in Physiology and Medicine from the University of Aberdeen and has a PhD in Medicine and Clinical Science from the Australian National University with specialty in cardiovascular medicine and haematology.
Professor Nair has received a number of awards from international organisations including being specially recognised for his role in coagulation research by the Australian Capital Territory government. He has run biotechnology companies in Australia, the US and Singapore.
Professor Nair has been heavily involved in mergers and acquisitions especially in the US and Europe and has US financial experience. Professor Nair’s corporate experience includes financial raisings, corporate public relations and organisational integration strategies, Human Resources, IP strategy and management, financial management and Research and Development.
In 2022 Professor Nair was conferred with an Adjunct Professorship with the University of Queensland and its Australian Institute of Bioengineering and Nanotechnology (AIBN).
Professor Nair is an international expert in Coagulation Science with a special emphasis on fibrinogen. Professor Nair is the author of over 100 research publications and has had over 50 patents on various aspects of electrophoretic plasma separation technologies, including the HaemaFrac® and their applications in both plasma protein separations, renal dialysis and cellular separations. He discovered the use of nano carbon particles in the radiographic imaging of blood clots.
Professor Nair is the principal inventor of the HaemaFrac® having developed and patented the plasma protein fractionation applications in the late 1990s to early 2000s. Professor Nair together with Mr John Manusu also ran the then world’s largest independent plasma collection organization based in the United States. This business collected both normal and specialty plasmas, including hyperimmunes for the US Government bioterrorism program.
Professor Nair has served on the boards of a number of international biotechnology companies and US state Commercialisation Boards for Georgia, Florida and Alabama. Professor Nair and Mr Manusu recently received cGMP certification for HaemaFrac®, the first new plasma fractionation technology anywhere in the world for the last 40 years of the industry.

Founding Managing Director
Mr John Manusu
Mr Manusu has over 30 years' experience running biotechnology companies worldwide. He has been involved in the development of the Aegros technology since its invention in 1985. Mr Manusu was also involved in the US plasma collection business creating, along with Professor Nair, the then world’s largest independent plasma collection organisation. This business collected both normal and specialty plasmas, including hyperimmunes for the US Government bioterrorism program. Throughout this period he has undertaken a number of significant cross border restructures, mergers, acquisitions and divestures. Finally, Mr Manusu has raised over $200 million in public funding and government R&D grants. Mr Manusu has a degree in Commerce and is a Fellow of the Financial Services Institute of Australasia. Mr Manusu has worked in the Australian, Asian and U.S. Biotechnology markets and is best described as a biotechnology entrepreneur.

Executive Director & Chief RAQA Officer
Ms Janet Bowen
Ms. Janet Bowen has almost 30 years of experience in the areas of sterile pharmaceuticals, biopharmaceuticals, oral solid dosages, and medical devices. During her career, she has held management positions in quality, regulatory, compliance, batch/lot release, and compliance/regulatory consulting. Ms. Bowen has worked for Sterling Drug, Sanofi, Abbott Laboratories, CAI Consulting, and PrIME Biologics prior to joining Aegros. She hosted numerous successful inspections from the FDA, EMA, CMDA, TGA, Health Canada. While she was with Sterling Drug and Abbott, the sites received approval of 30 plus new products over five (5) year time span. Many of them were generic drugs approved based on the data in the product dossier and without an onsite inspection. She has prepared companies for many different agency inspections, setting up quality systems, and to prepare the associated submissions. Ms. Bowen is knowledgeable in the requirements of FDA, HSA, EMA, CFDA, PMDA, TGA, and Health Canada, as well as other agencies. During her time in the industry, she has successfully registered over 50 products with the US FDA, EMA, TGA, HSA, and Canada. Ms. Bowen has a B.S. in Chemistry and a B. S. in Business Management. During her career, she has given presentations on Process Validation, Inspection Readiness, Combination Products, Quality by Design, and Aseptic Processing as well as others. She is a member of ISPE, PDA, ASQ, RAPS and is an active member of several regulatory Interest Groups within these organizations.
Aegros Strategic Advisory Committee

Chair
Mr Jan M Bult
Jan M. Bult brings over 45 years of relevant experience. After a start as a pharmaceutical sales representative (Ciba-Geigy and Schering AG), he worked as a product manager (Schering AG) and marketing manager (Rhone Poulenc) before he started to work in the plasma protein sector with BIOTEST AG.
For more than 21 years he was the President & CEO of the global Plasma Protein Therapeutics Association. You can really say that he has seen it all. Writing business plans, developing strategies, dealing with crisis management, handling media inquiries, testifying at congressional hearings, team motivation and training, making international presentations, you name it, and he has done it.
His network with regulatory agencies, medical experts and patient representatives is very impressive and he can open many doors that are closed for others. This network includes many persons and organizations in North America, South America, Europe, Asia and Australia.
Since 2019 he is active as consultant in the field and has consulted with various companies on different projects.

Member
Dr Erin Evans
Dr Evans is an experienced organisational and entrepreneurial leader with a strong focus on strategic partnerships and policy and innovation across multiple industries including biotech, healthcare, technology, defence, higher education and digital innovation. Highly effective at delivery of strategic programs building collaboration across multi-disciplinary teams and working with diverse, international stakeholders at all levels. Over 20 years on governance and advisory boards in Director and Chair roles.
Presently CEO of Life Sciences Queensland, Dr Evans has extensive periods of service on government, academic, industry, and medical panels and bodies.
Dr Evans has extensive networks of key stakeholders both nationally and internationally.

Member
Ms Gayle Ginnane
Ms Gayle Ginnane has 40 years’ experience in public sector management, regulation, policy review, multi-stakeholder engagement, audit and risk and board experience. Her experience includes chief executive, director and chair roles in both government and private sector primarily across the health and early childhood education sectors.
Ms Ginnane Chaired the National Blood Authority advisory board for 8 years as well as significant other governance experience in both the public and private sectors as the CEO of the health insurance regulator, a director on boards and chair of audit and risk committees in government, private and not-for-profit sectors.
Ms Ginnane served as CEO in a complex insurance and regulatory environment dealing with public and commercial sectors with small and large, including listed, companies. She has an in depth understanding of governance, risk management, the public private interface and experience in assessing the commercial realities in the business environment.
Aegros Global Management Team

CEO Aegros Engineering & Therapeutics
Mr Damian Thornton
Damian Thornton is Chief Executive Officer for Aegros Engineering and is responsible for the management of the Engineering organisation, developing business plans, budgets and strategies, identifying and managing operational and corporate risks for the organisation, leading the development of the company’s short- and long-term strategy, creating and implementing the Division’s vision and mission, evaluating the work of other executive leaders within the company.
He brings more than 30 years of international experience in the design, construction, Qualification and operation of Biopharmaceutical facilities, including Process design, optimisation, inter disciplinary design team management and multi-million dollar D&B project management and supervision.
Damian has more than 15 years of executive leadership with Executive and Operational experience in Asia, Europe, North and South America. He has experience in Business Strategy, Corporate expansion and transformation, Mergers & Acquisitions, Digital Strategy, Sales leadership and IPO.
Prior to joining Aegros, Damian held a variety of Senior Executive positions at international organisations such as CH2M Hill, M&W Zander, Commissioning Agents and Diaceutics.
Damian holds a Bachelor of Engineering degree from Queens University Belfast and is a member of Whos who in Engineering and Construction.

CEO Aegros Asia
Dr Ranjeet Ajmani
Dr Ranjeet Ajmani is Chief Executive Officer for Aegros Asia.
Dr Ajmani is called the father of India’s blood plasma industry and has a well-established and respected reputation across South-East Asia for his academic, commercial, and policy work setting up blood transfusion and blood plasma collection networks and infrastructure.
He holds a Doctorate in Biomedical Engineering from the Indian Institute of Technology (IIT), Mumbai, and he was a Visiting Fellow at the National Institutes of Health (NIH), USA. He has delivered academic and industry presentations on various issues related to Plasma Protein Therapy, especially in reference to developing and under-developed countries, at both National and International meetings organized by PPTA, IPFA, AATM, ISTM, ISBT, and Bio-Plasma Asia.
Dr Ajmani has authored chapters in plasma fractionation monographs, is a visiting faculty member of academic and research councils at several institutions. Dr Ajmani is an Advisor to the Plasma Proteins Therapy working Group of the Asian Association of Transfusion Medicine (AATM). He is also member of the Expert Committee on blood plasma products of the Indian Pharmacopeia Commission (IPC).
In his spare time, Dr Ajmani is a passionate teacher and mentor, a prolific speaker, a creative writer, cook and an influencer.

Chief Capital Officer
Mr Alex Stuke
Alexander Stuke is Chief Capital Officer.
He brings more than 35 years of international cross-functional experience in Finance, Mergers & Acquisitions, Digital Strategy, Business Transformation & Innovation, Robotics Process Automation, Advanced Analytics and Corporate Advisory.
Prior to joining Aegros, Alexander held a variety of Senior Executive positions at organisations such as Commonwealth Bank of Australia, Qantas Airways, AMP Group, GE Capital (Australia & NZ), GE Capital (Europe), General Electric Industrial Systems (Europe), QBE, BlackRock, Ernst & Young, Vodafone, Pulse Markets, and NSW Office of State Revenue.
His industry experience covers Financial Services, Investment Management, Capital Markets, Banking, Insurance, Aviation, Big-4 Consulting, Telecommunications, Manufacturing and Public Sector.
Alexander holds a Bachelor of Science degree from University of NSW, Graduate Diploma in Applied Finance and Investments, is a member of Chartered Accountants Australia & NZ, Fellow of CPA Australia, Fellow of Finance & Insurance Institute of Australasia, Senior Associate of Australian & NZ Institute of Insurance and Finance and a member of Australian Institute of Company Directors.
Alexander is an Australian Citizen, lives in Sydney with his wife, two sons and two Maine Coon cats.

Global Chief Science & Technology Officer
Emeritus Professor Stephen Mahler
Emeritus Professor Stephen Mahler recently retired as the Director of the Australian Research Council (ARC) Training Centre for Biopharmaceutical Innovation and Deputy Director of the National Biologics Facility, Queensland Node.
He was also a Senior Group Leader at the Australian Institute for Bioengineering and Nanotechnology (AIBN), and Professor in the School of Chemical Engineering, The University of Queensland. He has been involved in the research and development of biologic medicines for over 25 years.
He was appointed to the AIBN in January, 2007.

Chief Supply Chain Officer
Mr Leighton Hopper
Leighton Hopper is Chief Supply Chain Officer for all of Aegros’ operations.
Leighton Hopper has both an Honours degree in Organic Chemistry and an MBA in Strategy Innovation. Leighton’s background includes senior management positions both in Australia and internationally in the pharmaceutical, chemical & FMCG industries including CPSA, Univar, Selleys and Dulux.
He was the CEO and CFO of CPSA, acquired by Aegros and has experience in the management of finance, strategic funding, working capital, supply chain & operations.

Chief Sales & Marketing Officer
Mr Mike Swan
Mike Swan joined Aegros in 2023 as Chief Marketing Officer. Mike has had a 25-year career in the biopharmaceutical industry spanning several specialty therapeutic areas inc. infectious diseases (HIV, Hepatitis, Antibiotics, and Invasive Fungal Infections), haematology, immunology, and rare diseases.
Prior to Aegros, Mike held commercial positions with Merck and Co., Baxter, Gilead and Novartis. During his 9 years at Merck, he held several leadership roles in Asia, and led the launch of ZEPATIER for Hepatitis C across the Asia-Pacific region. He has also led the successful launches of numerous specialty pharmaceutical brands in the UK, Asia, Australia, and NZ, in haemophilia, HIV, Hepatitis B, anaesthesia, cardiology, and intensive care.
Mike is a graduate of the Strathclyde University Pharmacy School and began his career as a retail Pharmacist in the UK, before beginning his career in industry at Roche Products UK.

Chief Medical Officer
Dr Leon Rozen
With more than 25 years’ experience in global medical affairs and medical communications, Dr Rozen has shown medical leadership of launches across therapeutic areas at global, regional and affiliate levels, while interacting with clinical and commercial stakeholders as a medical science liaison.
Dr Rozen has had a number of roles in the industry including as Medical Director of the Pharmaceutical Division of CSL and Vice President of Global Medical Affairs and Immunology at Baxter in Chicago. Most recently he was Medical Director ANZ for Ipsen, a global pharmaceutical company focused on transformative medicines in oncology, rare disease and neuroscience.
Dr Rozen, a trained haematologist, holds a Bachelor of Medical Science and a Doctor of Medicine from Monash University, and is a fellow the Royal College of Medical Administrators.

General Counsel & Company Secretary
Dr Shanta Nair
Dr Nair started out studying medical science at Macquarie University, where she specialised in medical genetics and immunology. Through a collaboration with
Singapore’s National University, she published a paper with Professor Hari Nair on the expression of novel immune system proteins in human blood in 2014 during her undergraduate studies, going on to complete a Master’s thesis in immunology and genetics.
She then pursued a doctorate in law, where her dissertation focused on the intersection between medicine and law and the new space opening up in biotechnology regulation. Dr Nair had the opportunity to study advanced corporate law and to gain practice in the field, working under a barrister in
the United Kingdom and clerking at HWL Ebsworth before qualifying as a practicing lawyer. She has previously worked for the Federal Department of Health and at PriceWaterhouse Coopers, before joining Aegros in 2021.

Chief Financial Officer
Mr Tom Milicevic
With more than 25 years’ experience with major local and international organisations, Tom brings significant finance and operational experience developing and executing business strategy, IT, operational and financial performance while managing stakeholder needs and working collaboratively across functions.
Tom’s previous experience has included roles with major local and international organisations including Cochlear Limited, Babcock & Brown as well as start-up and early-to-late-stage companies. Tom has been responsible for several capital initiatives including Initial Public Offerings and secondary raisings across Healthcare/Medtech, Manufacturing, Construction & Infrastructure, Engineering and Property.
Tom holds a Bachelor of Commerce (Law) from Western Sydney University, Fellow of CPA Australia, a Masters in Business Administration from Macquarie University and is Graduate of the Australian Institute of Company Directors.
Scientific Advisory Committee

Chair
Emeritus Professor Stephen Mahler
Emeritus Professor Stephen Mahler recently retired as the Director of the Australian Research Council (ARC) Training Centre for Biopharmaceutical Innovation and Deputy Director of the National Biologics Facility, Queensland Node. He was also a Senior Group Leader at the Australian Institute for Bioengineering and Nanotechnology (AIBN), and Professor in the School of Chemical Engineering, The University of Queensland. He has been involved in the research and development of biologic medicines for over 25 years. He was appointed to the AIBN in January, 2007.

Committee Member
Professor Susan Shafstein
Susan Sharfstein is a Professor of Nanobioscience at SUNY Polytechnic Institute in Albany, New York. Professor Sharfstein received her B.S. in chemical engineering with honors from Caltech in 1987 and her Ph.D. in chemical engineering from UC Berkeley in 1993, receiving graduate fellowships from the university and the National Science Foundation. She received a National Institutes of Health Individual Research Service Award to pursue postdoctoral studies, initially at UC Berkeley and subsequently at the UCLA Medical School.
Dr. Sharfstein joined the faculty at the University of Toledo in Bioengineering in 1996. In 2000, she received a National Science Foundation POWRE award to study glycobiology at the New York State Department of Health Wadsworth Laboratories. In 2001, she joined the Department of Chemical and Biological Engineering at Rensselaer Polytechnic Institute, and in 2007, she received a dual appointment in Biology. In 2010, she joined the faculty at SUNY Polytechnic Institute, College of Nanoscale Science and Engineering.
Professor Sharfstein received an NSF CAREER grant in 2000 for her work on hyperosmotic stress responses of hybridoma cells and the School of Engineering Education Excellence Award and the Class of 1951 Outstanding Teaching Award in 2007. Professor Sharfstein’s research interests include mammalian and microbial cell bioprocessing, control of protein glycosylation, metabolic engineering, biosensing, and development of systems for high-throughput screening of nucleic acids and small molecules. Professor Sharfstein served as the subject editor for biotechnology for the Elsevier Major Reference Work Life Science Module from 2015-2018. She is the author of over 70 papers and book chapters in the fields of biotechnology and bioprocessing. She was a 2017-18 recipient of a Fulbright Global Scholar award and spent her sabbatical at Dublin City University, in Dublin, Ireland and University of Queensland in Brisbane, Australia performing proteomic analysis of Chinese hamster ovary cells and studying bispecific antibodies. She currently serves as a Fulbright Alumni Ambassador in addition to her research and teaching activities.

Committee Member
Professor Kris Thurecht
Prof. Kris Thurecht is a joint Senior Group Leader at the Australian Institute for Bioengineering and Nanotechnology (AIBN) and Centre for Advanced Imaging (CAI), and is currently the Deputy Director, Imaging Technologies at CAI and Director of the ARC Research Hub for Advanced Manufacture of Targeted Radiopharmaceuticals, University of Queensland.
His team works across the boundaries of chemistry, biology and imaging science to understand how materials interact with biology, and then use this information to develop new pharmaceuticals.
Prof Thurecht’s research focusses on the development of polymer and nanoparticle-based devices for nanomedicine. For polymers to be truly effective in nanomedicine, they must incorporate new therapies while maintaining their physical and chemical integrity. This can only be achieved by developing a strong understanding of the fundamental properties of the nanomaterial-delivery system, in addition to identifying and successfully delivering new therapies.
Central to the development of these future therapeutic platforms, is the field of theranostics, where molecular imaging plays a key role in understanding the dynamics of polymeric nanomedicines.

Committee Member
Professor Kathryn Uhrich
My research focuses on the design, synthesis and characterization of biocompatible, biodegradable polymers that serve a critical need in therapeutics/drug delivery.
My most well-known invention is “PolyAspirin”; it was the first example of a polymer that degrades into a bioactive such as salicylic acid (SA) that can locally reduce inflammation and pain.
These polymers were evaluated for various wound healing applications, such as promoting bone regeneration in diabetic animals. Another invention is the amphiphilic macromolecules (AMs), which are also bioactive. The bioactive AMs were unique therapeutic coatings for metal cardiac stents to reduce smooth muscle cell (SMC) proliferation and platelet adhesion in humans. To date, this research has produced more than 190 peer-reviewed papers and generated nearly $30 million in federal and corporate research funding.
In entrepreneurship and technology transfer, I’m an inventor on more than 70 U.S. and international issued patents based upon research performed in my research labs. I’ve also been involved in multiple start-up companies centered on biomedical polymers and worked in close partnership with companies, such as BASF, Chanel, Colgate-Palmolive, DuPont, ExxonMobil, and L’Oréal.
The partnerships are typically to develop new materials and/or products that are nontoxic and biodegradable that meet an unmet consumer need in pharmaceutics, medical devices, personal care, and environmental applications.
Encouraging curiosity, brainstorming, preparing for failure, and promoting teamwork are keys to the success of my research labs.
Our History
We trace our roots back to Dr Joel Margolis who escaped out of Poland as WWII was erupting in September 1939. Joel migrated to Australia with his family and read medicine at Melbourne University before becoming a preeminent researcher in the haematology field. Joel was the first person to develop a process to make FVIII AIDS free in early 1980’s. Joel invented the tangential flow Electrophoresis process as an alternative to NASA’s attempts to do biological separations in space.
Dr Perry Manusu, an Australian Veterinary, Surgeon and entrepreneur, who created the largest vet practice and vet pharmaceutical company, joined with Joel to develop this process which they called The Gradiflow. The first Gradiflow patent was lodged in 1984.
In 1985 John Manusu joined the company and helped take Gradipore public in May 1986. Tangential flow Electrophoresis as applied to plasma protein purification was spearheaded by Dr Hari Nair when he joined Gradipore in 1998 and developed the various applications of the Gradiflow including viral and prion removal.
By early 2002 there were some 50 patent families covering a number of aspects of the Gradiflow technology. In 2004 the 3 founders (Perry, Hari, John) rebuilt Gradipore and acquired Serologics, a US plasma collection business specialising in hyperimmune plasmas through FDA approved centres. They used this acquisition to refine the plasma fractionation process, particularly for hyperimmune products. By 2007 the company, then called Life Therapeutics, was the largest non-fractionator plasma collection business in the world, collecting normal, hyperimmune and bioterrorism plasmas. They agreed to sell the business to the Italian fractionator Kedrion. Having announced this agreement the founders retired, and the new Board sold the collection centers.
In the meantime, John and Hari setup another company called NuSep which developed an IVF application for the Gradiflow technology. A successful clinical study was completed in 2008. In 2010, NuSep was offered a small cGMP facility in Singapore and the company chose to reactivate the plasma separation technology. Hari further refined the fractionation process particularly around the disposable cartridge which enabled the processing of plasma collected from emerging economies.
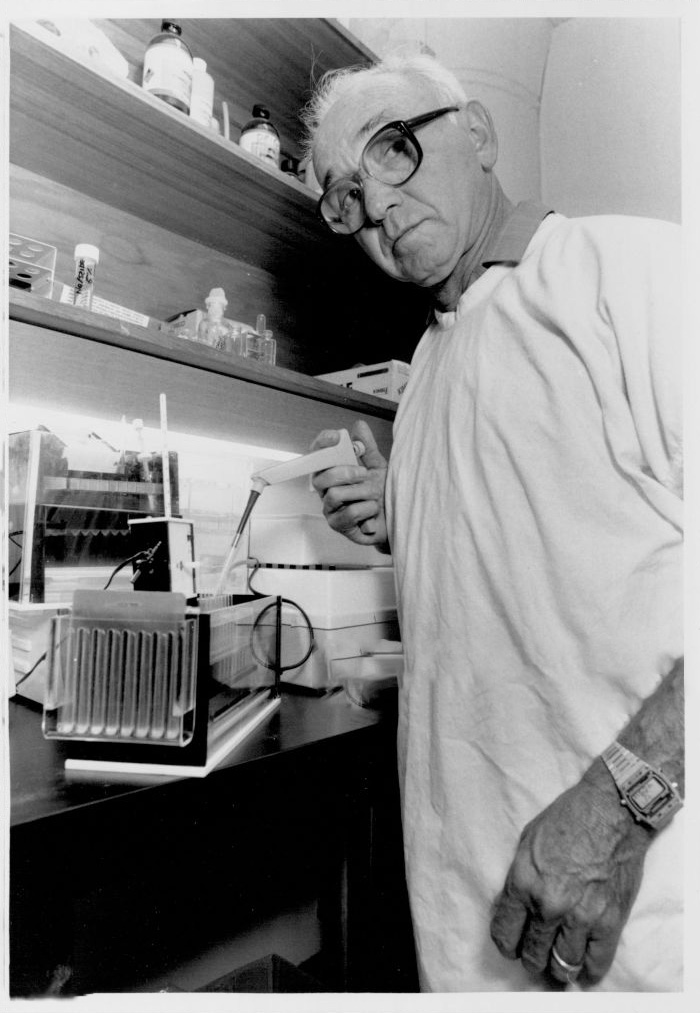
Dr Joel Margolis
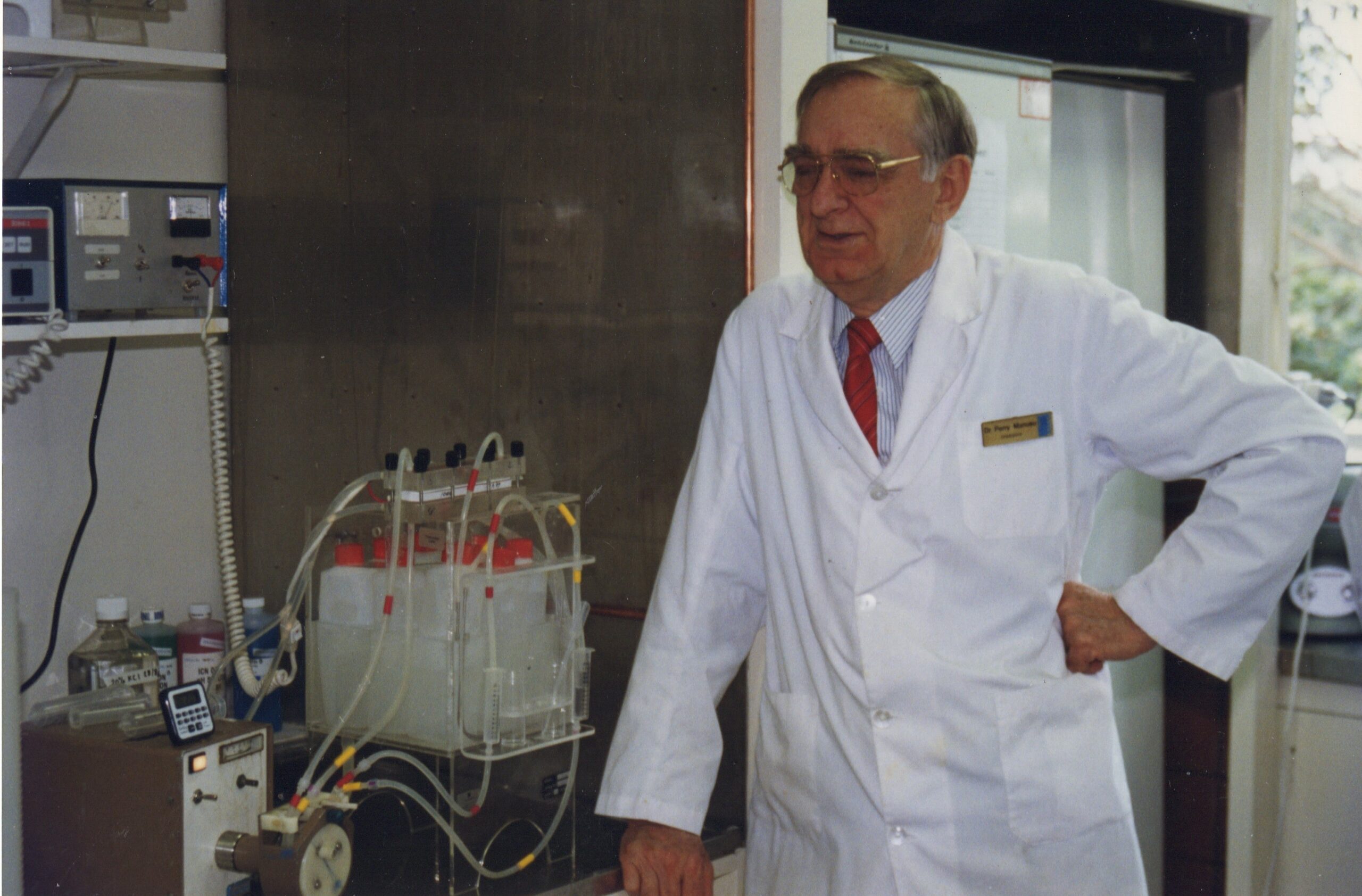
Dr Perry Manusu

Perry and Joel working together with the original Gradipore system

The original Gradipore HQ
In 2013 this business was spun out into a company called PrIME Biologics and was funded by private equity. On 1st July 2016 PrIME Biologics obtained cGMP registration from the Singapore Health Sciences Authority for the fractionation of Albumin from human plasma. This was the critical regulatory approval necessary to enable this process to be used to manufacture a therapeutic plasma product. This was also the first time that a regulatory authority had granted GMP status to a company not using conventional Cohn/chromatography for plasma fractionation.
Hari and John left PrIME Biologics in April 2017 after it became clear funding of the commercial scale plasma fractionation facility could not be achieved within PrIME Biologics.


In May 2017 Hari and John established Aegros. Over the next two years Hari developed the HaemaFrac® process to fractionate hyperimmunes from plasma.
In 2013 this business was spun out into a company called PrIME Biologics and was funded by private equity. On 1st July 2016 PrIME Biologics obtained cGMP registration from the Singapore Health Sciences Authority for the fractionation of Albumin from human plasma. This was the critical regulatory approval necessary to enable this process to be used to manufacture a therapeutic plasma product. This was also the first time in the last 40 odd years that a regulatory authority had granted GMP status to a company not using conventional Cohn/Chromatography for plasma fractionation.
Hari, John and Kailing left PrIME Biologics in April 2017 after it became clear funding of the commercial scale plasma fractionation facility could not be achieved within PrIME Biologics.

